Which Statement Best Describes the Ph of Pure Water
3 5 pts Question 15 1451 1184 1250 168 1232 A 480 g sample of. It is acidic because it has a hydronium ion concentration of 10 x 10-7 M.
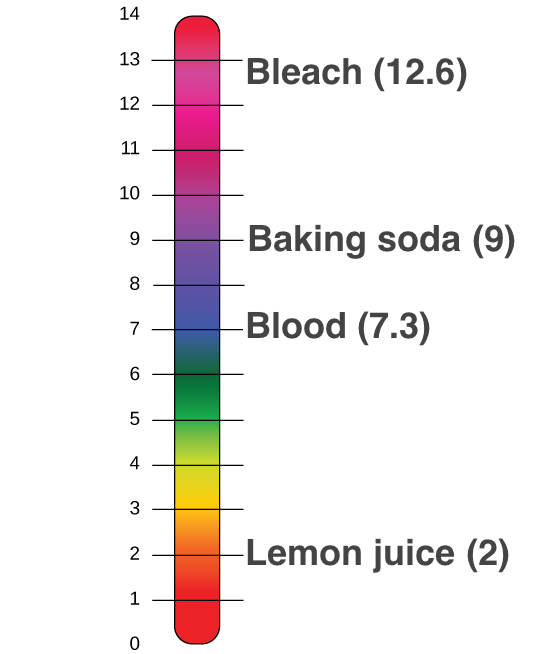
Ph Acids And Bases Review Article Khan Academy
Water self-ionizes to form an equilibrium system that has H3O 10 10.
. Which statement best describes the pH of pure water. 14 In regard to water which of the following is the best example of adhesion. Water molecules are made of atom b.
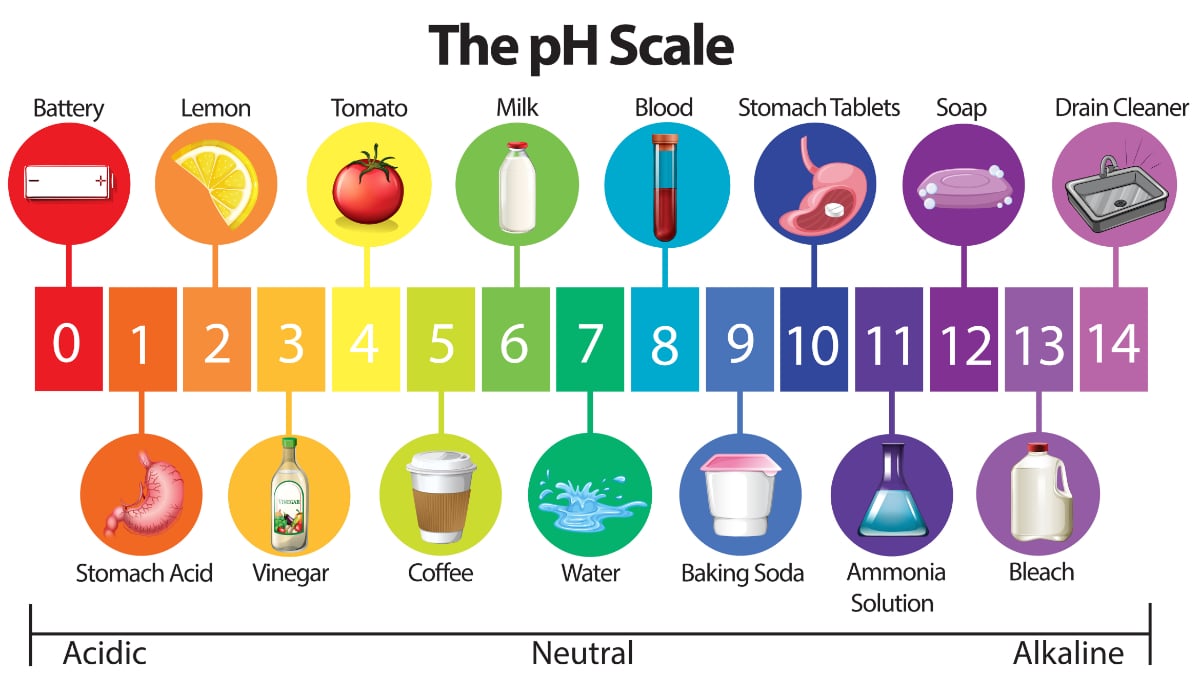
Which statement best describes the pH of pure water. The pH scale shows how acidic or how basic these compounds are. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions.
The higher hydronium ion concentration tends to move the pH from 7 towards. Water molecules are attracted to each other d. The table compares the number of electrons in two unknown neutral atoms.
Water molecules are wet. It is neutral because the concentration of hydronium ions equals that of hy. Which is a non-example of matter.
Which statement best describes ph of pure water Other questions on the subject. When the value of pH is less than 7 then the solution is acidic. It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
Which statement best describes PH of pure water 1 See answer phatgirl2001ori0rr is waiting for your help. B A water strider runs across a pond without breaking the surface. BIt is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions.
Pure substances maybe homogeneous or heterogeneous. They immediately Ine and become one drop. The answer is letter A.
PH is equal to the negative log of hydrogen. Pure water has a 614 pH in temperatures that are at 100 degrees celsius. Sodium hydroxide a compound commonly found in drain cleaners has a pH of 13.
Water molecules are attracted to each other c. It is neutral because the concentration of hydronium ions equals that of hydroxide ions. Hence the best statement for the pH of pure water is It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
Which of these statements best explains why pure water has a pH of 7. It is neutral because the pure liquid contains neither hydronium ions not hydroxide ions. View the answer now.
Smenevacuundacy and 14 more users found this answer helpful. The higher the pH the more basic it is. 5 pts Question 14 1000 times more 30 times more 3 times more 1000 times less 300 times less The hydronium io n H O concentration in a solution with p H 10 is _____ than the hydronium ion concentration in a solution with p H 13.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions. The statement which best describes the pH of pure water is the first one among the choices. Which statement best describes the pH of pure water.
It is a form of energy. Anything higher than 7 would make water acidic already. Which best explains this.
AIt is neutral because the concentration of hydronium ions equals that of hydroxide ions. It has a definite shape. Water molecules are magnetic d.
Which statement best describes the pH of pure water. This just means that pure water is in the neutral zone of the pH scale. The pH of water is 7 which equal to 10-7 of H and OH- ions.
Report 3 digits after the decimal. Which statement best describes the pH of pure water. When the value of pH is equal to 7 then the solution is neutral.
C Island climates tend to be more moderate than those inland. CIt is acidic because it has a hydronium ion concentration of mc027-1jpg. The pH of the solution is to the third decimal place Is this solution acidic.
Chemistry 22062019 0730 10040813. Which of the following best describes matter. D Water spilled on a table form a drop rather than spreading out.
It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. The pH of a solution decreases by 20. Tue May 30 2017 Which statement best describes the pH of pure water was asked on May 31 2017.
The pH of water is 7 which is equal to 10-7 of H and OH- ions. When you bring two drops of water near each other and allow them to touch. It is acidic because it has a hydronium ion concentration of 10 times 10 to the negative 7 moles per liter.
The lower the pH the more acidic a compound is. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. How does the hydronium ion concentration of the solution change.
Question 10 1 pts Calculate the pH of pure water at 45 C Kw 420 x 1014 at 45 C. The pH of pure water is neutral because the concentration of hydronium ions equals that of hydroxide ions. It is basic because it has a hydroxide ion concentration of.
Which statement best describes the ph of pure water. It is acidic because it has a hydronium ion concentration of mc027-1jpg. It is acidic because.
QA Chemistry Which statement best describes the pH of pure water. Zandy2 zandy2 The concentration of hydronium ions equals that to hydroxide ions the answer is A pH is equal tot he negative log of hydrogen. If water reaches a 7 pH scale it is considered as an alkaline water.
A Water molecules cling to each other. Which statement is TRUE regarding pure substances. Add your answer and earn points.
Comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. One word answer Ideas Question 3 1 pts For each region of the diagram below A-E match the statement that best describes the molecular scale. PH 7 has been the neutral pH.
The solution is. When the value of pH is more than 7 then the solution is basic. It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
The pH of pure water has been best described as neutral pH with equal hydronium and hydroxide ionsThus option A is correct. PH has been described as the measurement of hydrogen ions in a solutionThe pH has been measured on a scale of 1-14. Which of these statements best describes the contents of the pile.

Ph Pure Water Products Llc Beauty School Cosmetology Hair Science Cosmetology Student

Ph Of Pure Water Specialized Sensors Increase Measurement Performance

Best Truck Driving Jobs Images On Pinterest Driving Truck Driving Jobs Trucks Truck Driver Jobs

Which Statement Best Describes The Ph Of Pure Water A It Is Neutral Because The Concentration Of Brainly Com

Lemon Oil Eye Cream Diy Recipe Invivo Essential Diy Eye Cream Homemade Eye Cream Essential Oil Cream
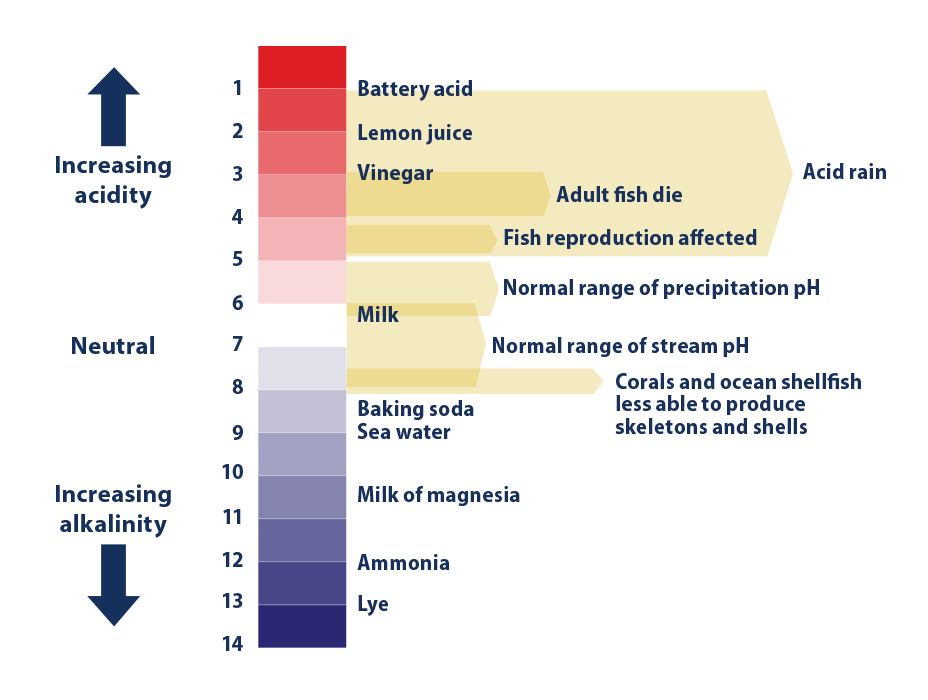
Climate Change Indicators Ocean Acidity Us Epa

Klar Seifen Inc Video Video In 2021 Natural Soaps Recipes Palm Oil Free Soap Natural Cosmetics Diy

Ph Of Pure Water Specialized Sensors Increase Measurement Performance
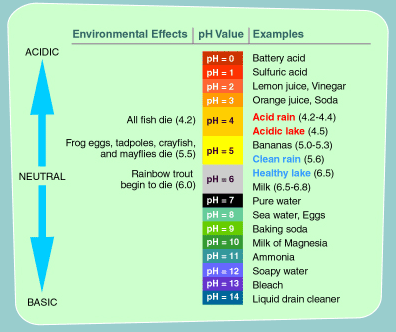
Acid Rain Students Site Ph Scale

Los Angeles Ca Artist Fawn Rogers Photography Themes Reflection Photography Visible Light

Lemon Myrtle Fragrances Face And Body Moisturiser 500ml Lemonmyrtlefragrances Fragrance Body Moisturizer Face And Body

Swallow This Fall Asleep Almost Instantly Stay Asleep And Wake Up Refreshed Alkaline Water Benefits Drinking Alkaline Water Alkaline Water

Pin By Rollsgabriella On Moa 2 Video Prom Dresses Dresses Formal Dresses

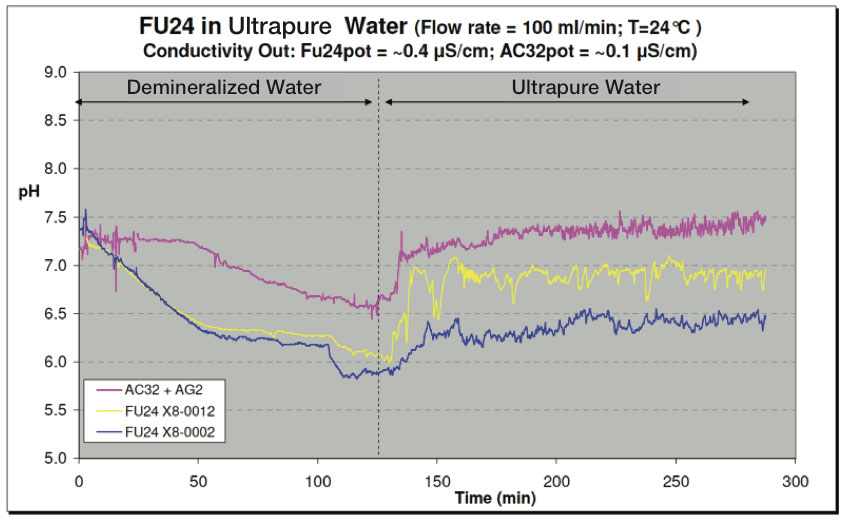
Understanding Ultrapure Water And The Difficulties With Ph Measurement Yokogawa Electric Corporation

Ph Of Pure Water Specialized Sensors Increase Measurement Performance



Comments
Post a Comment